
Informed Consent Process Redesign
The Challenge: The overall objective of this proposal is to use design thinking methodologies to develop and test a novel Informed Consent Document (ICD) in the emergency department that: 1) reduces the length; 2) decreases the complexity; 3) improves the visual appearance; and 4) increases participant understanding and recall of the ICD process.
The studies involve several critical aspects requiring consent, including specimen collection and retention of stored specimens, in the busy ED setting including a population of acutely ill children.
The Team: Blake Lane - PhD Design Research Fellow; Hannah Eber - Industrial Design Co-op; Lauren Goodwin - Graphic Communication Co-op; Becca Nachtrab - Graphic Communication Co-op; Kylie Boyd - Fashion Design Co-op
The Timeline: January 2017 - May 2017



8 clinical staff, 4 families, and 20 hours of observations were completed to understand all the touch points and stakeholders of the informed consent process in the emergency department. Interviews with staff and families highlighted opportunities and challenges with the current process.
A list of items that “must be true” of a new process was created by understanding the opportunities and challenges of the current system. This list acted as the design criteria for the final intervention.

The proposed informed consent toolkit seamlessly integrates six interventions into the ICD process. Each intervention touches different phases of the ICD journey. The system includes an ICD Research Portal for researchers to populate their ICD’s. The portal prints out a consolidated ICD. The CRC’s would use a video to explain what research is for patient and families, and supplement that information with educational brochures and pamphlets on that particular study. To expedite the ICD process, the CRC would use an app, along with flexible status signs for the patient rooms to notify any clinical staff where the patient and/or family is within the ICD process. The following is the proposed informed consent journey and the six different conceptual interventions.


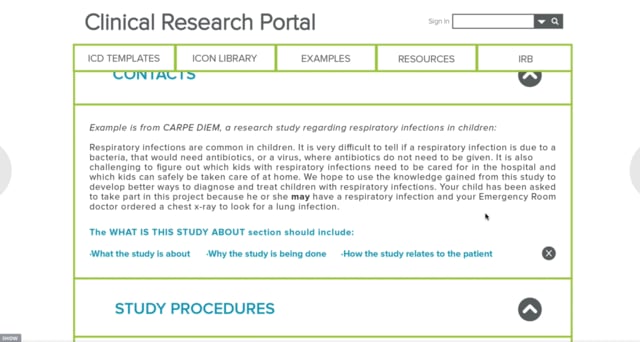





Use the link below to access the InVision prototype:
https://projects.invisionapp.com/share/2MBBNJ9SK#/screens/229274989_Artboard_1-100

The interventions have not made it out of concept stage but are being considered for future innovations within the hospital.
Credits to: Todd Florin, MD; Lilliam Ambroggio, PhD; Robert Frenk, MD; Jackie Grupp-Phelan, MD; Stephen Porter, MD